Structure and Function of Semiconducting Polymers
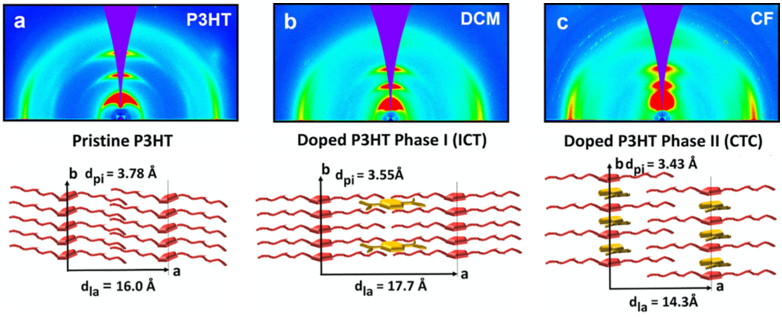
2D grazing-incidence wide-angle X-ray scattering (GIWAXS) patterns of pristine and F4TCNQ-doped P3HT thin films and corresponding cartoons illustrating how F4TCNQ¬— anions can incorporate into a P3HT crystallite (Stanfield et al., 2021). By tracking changes in crystallite spacings during doping, different structural changes in semiconducting polymers crystallites can be correlated with the formation of mobile charge carriers (polarons/bipolarons) to identify the impact of different charge transfer modes (e.g., integer charge transfer (ITC) vs charge-transfer complex (CTC)).
Our primary work in this area focuses on the structure of doped semiconducting polymers (SCPs) and conjugated polyelectrolytes (CPEs) as a collaborative effort between the Tolbert Lab, Schwartz Lab, and Rubin Lab at UCLA, in partnership with other universities and synchrotron light sources. Work from the Tolbert group falls into two main categories — the first is the study of the structural changes induced in semiconducting polymers (P3HT, PBTTT, ProDOT, etc.) by chemical and electrochemical doping. The goal of this work is to understand how nanoscale changes in polymer structure correlate with anion shape/size and how the distribution of anions in the crystallite lattice can induce the formation of trapped or mobile charge carriers and affect macroscopic film conductivity. The second category of work is on the structural and spectroscopic characterization of cylindrical micelle-forming self-assembling conjugated polyelectrolytes. These water-soluble semiconducting polymers are highly solution-processable and serve as a model system for spectroscopically probing the chemical doping of semiconducting polymers in solution at dynamic equilibrium.
Useful References
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
